 Athletes who want to build muscle mass can use not only proteins, creatine and amino acids, but also fish oils to achieve this. The anabolic effect of omega-3 fatty acids has been confirmed, so now molecular scientists at the University of Aberdeen have gone a step further. They are about to publish the results of a study in Biochemical and Biophysical Research Communications from which you can deduce which fish oil supplements have the strongest anabolic effect: those containing lots of EPA.
Athletes who want to build muscle mass can use not only proteins, creatine and amino acids, but also fish oils to achieve this. The anabolic effect of omega-3 fatty acids has been confirmed, so now molecular scientists at the University of Aberdeen have gone a step further. They are about to publish the results of a study in Biochemical and Biophysical Research Communications from which you can deduce which fish oil supplements have the strongest anabolic effect: those containing lots of EPA.
Taking fish oil supplements or eating a diet that is rich in fish fatty acids have a body recompositioning effect: your fat mass decreases and muscle mass increases. Scroll down to find links to articles on the anabolic and fat-cell killing effect of fish fatty acids.
Researchers in Aberdeen, Scotland, were curious about how fish fatty acids can have an anabolic effect, so they designed experiments with full grown C2C12 muscle cells from mice to try and work out the mechanism involved. They exposed the cells in test tubes to 50 micromols of the fish fatty acids DHA and EPA, and stimulated anabolism with leucine.
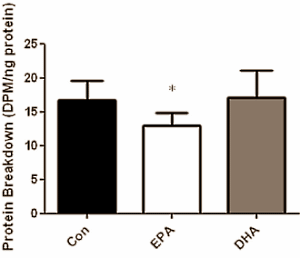
When the researchers measured the build-up and breakdown of muscle cell protein, they noticed that these were 25 percent higher and 22 percent lower respectively in the muscle cells that had been exposed to EPA.

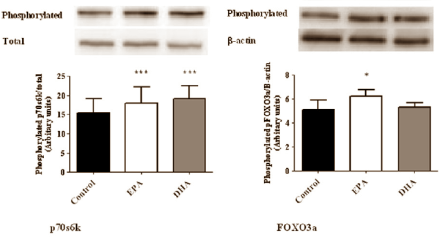
The researchers then looked at the activity of anabolic signal molecules in the muscle cells. The figure below shows that EPA and DHA both activated p70s6k, but only EPA activated FOXO3a. The fish fatty acids had no effect on the other signal molecules that the researchers examined: Akt, mTOR, 4EBP1 and rps6.
"Fish oil supplementation containing a higher proportion of EPA than DHA could be the most efficacious in improving protein accretion in response to anabolic stimuli such as L-leucine/resistance exercise and could attenuate protein breakdown in ageing skeletal muscle", the researchers conclude. "Further work in humans is clearly required to test this hypothesis."
Source:
Biochem Biophys Res Commun. 2013 Mar 22;432(4):593-8.

Comments